Term Used to Describe the Amount of Soute Per Solvent.
Concentration is a measurement of the amount of solute present in a chemical solution with respect to the amount of solvent. It is important to distinguish between three closely related terms solute solvent and solution.

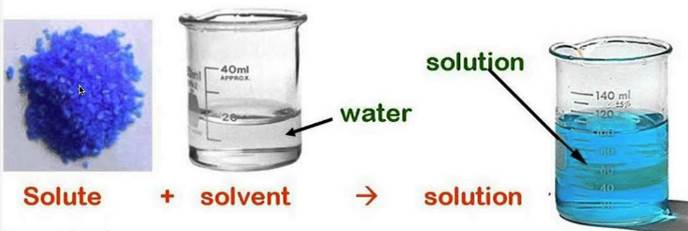
Solute Solvent And Solutions Diagram Quizlet
A solvent is usually a liquid.
. The amount of solvent required depends strongly on the extraction mode used. The terms solute and solvent are really only meaningful when one is much more than the other. A solute is a substance that can be dissolved by a solvent to create a solutionA solute can come in many forms.
Main characteristics of a solution Solution is homogenous. The term used is solubility. Which of the following statements is true about.
If you know the molarity concentration of a solution and the total volume of the solution make sure it is in liters you can determine the number of moles. Characteristics of a solution are identically distributed through it. The major proportion of the solution remains occupied by the solvent.
The solvent or substance that dissolves the solute breaks the solute. Which term is used to describe the amount of solute in a solution. Pages 22 This preview shows page 21 - 22 out of 22 pages.
Solubility is the capacity of a substance to dissolve in a given amount of solvent. Your email address will not be published. A solute can take many forms.
There are several ways to determine the amount of solute in a solution. You cannot differentiate one substance from another within the solution. HCl can dissolve zinc metal through a process of displacement with the result of the displacement.
Formed one substance dissolves into another. But concentration units always describe the ratio of the amount of solute in a sample to the amount of either the solvent or the solution. For an easy example if you.
Harvard University. It can be gas liquid or solid. Which term is used to describe the total amount of a solute that can dissolve into a particular solvent.
It can break the molecular interaction between solute-solute molecules and suspend the free solute molecules evenly to make a solution. Which term is used to describe the total amount of a solute that can dissolve into a particular solvent. You might be interested in.
Course Title CHEMISTRY 206. Click here to get an answer to your question which term is used to describe the amount of solute in a solution amybofo12 amybofo12 4 weeks ago Biology College answered Which term is used to describe the amount of solute in a solution 1 See answer amybofo12 is waiting for your help. Substances mixtures and solubility.
The substance in which the solute is dissolved is called a solvent. The material present in the smaller amount in the solution. Examples of Solutes Usually a solute is a solid that is dissolved into a liquid.
This is because its amount is always greater than that of the solute. The equation for molarity is. Add your answer and earn points.
A solute is a substance that can be dissolved into a solution by a solvent. The rate of solution describes how fast a solvent dissolves a solute while solubility is the maximum amount of a substance that can be is dissolved. A solution containing the maximum amount of soute that will dissove in its solvent.
Alcohol and water are fully miscible in each other. In the static mode the sample is extracted with a minimum volume of solvent usually solvent volume used in the static mode does not ensure quantitative. Solute concentration is a term used to describe mixtures and defines how much of one substance called the solute is dissolved in another referred to as the solvent.
Solubility is also not equivalent to a substances ability to dissolve another substance when prompted by chemical reactions. What is the term used to describe the maximum amount. The solvent is that component of the solution which dissolves the solute.
An everyday example of a solute is salt in water. Osmolarity is defined as the number of osmoles of solute per liter L of a solution. Because so many calculations are based on the concept of the mole the most commonly used concentration unit is based on the number.
Students who viewed this also studied. A homogeneous mixture consisting of a solute dissolved into a solvent. An unusually strong dipole-dipole attraction occurring between molecules that have a hydrogen atom covalently bonded to a small highly electronegative atom usually nitrogen oxygen chlorine or flourine.
What is the term used to describe the amount of solute dissolved in a specific amount of solvent or solution. At a particular point called as equilibrium point no more solute can dissolve in the solventdifferent solute dissolve differently depending on their solubility value it is given in terms of given amount of solute in per 100 gram of solvent. What is the term used to describe the maximum amount of solute that a given.
That means you can form a solution at any concentration from very small proportion alcohol up to pure alcohol. Osmolarity depends on the number of particles in a chemical solution but not on the identity of those molecules or ions. It may be in the form of a gas a liquid or a solid.
A homogeneous mixture composed of two or more substances in which a mixture a solute is a substance dissolved in another substance known. Salt is the solute that dissolves in water the solvent to form a saline solution. The part of a solution that is present in the greatest amount is called a solvent.
When you have a very small amount of alcohol in water its easy to decide that the water is the solvent and alcohol is the. One best example of solute in our day to day activity is salt and water. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution.
Terms in this set 15 solubility. It is expressed in terms of osmolL or OsmL. A concentrated solution is one that has a relatively large amount of dissolved solute.
Evaporation distillation dissolving point solubility. Sample Osmolarity Calculations A 1 molL NaCl solution has an osmolarity of 2 osmolL. The material present in the larger amount in the solution.
There are a number of ways to describe concentration depending on need and can involve weight volume or molecular mass. The term used to describe how much solute dissolves in a given amount of solvent. Salt dissolves in water and therefore salt is the solute.
One method is using molarity most often method used in chemistry. Its the liquid that the solute is dissolved in. Describes a solution that contains a large amount of solute per given amount of solvent.
That the solution is a homogenous mixture means that it forms a single phase. Luque-García in Encyclopedia of Analytical Science Second Edition 2005 Solvent Volume. Leave a Reply Cancel reply.
Molarity moles of soluteliters of solution. See answer 1 Best Answer. The concentration of mixtures is important because many chemical reactions.
In fluid solutions the amount of solvent present is greater than the amount of solute.

Solutions And Solubilities All Solutions Have At Least Two Parts Solute Solvent Part Of Solution Present In Largest Amount Substance That Is Dissolved Ppt Download

Vocabulary In Solution We Need To Define The Solvent The Component Whose Physical State Is Preserved When Solution Forms Solu Solutions Physics Concentration
0 Response to "Term Used to Describe the Amount of Soute Per Solvent."
Post a Comment